Home
> Research
> Magnetic Field in Ion Exchange
Investigating the Effects of Magnetic Fields on Ion Exchange
Processes
Magnetic Field Effects
In 2001,
I began working with Profs.
Arthur Kney and
Javad Tavakoli to study
the behavior of an ion-exchange water purification system in the presence
of an applied magnetic field.
A back-of-the-envelope calculation demonstrates that the thermal energy
is much larger than energy of a magnetic moment in any reasonable
applied magnetic field. However, susceptibility measurements confirm
that, even in solution, there is still a small tendency of the magnetic
moments to align, completely consistent with what one would expect from
Curie's law.
Whether this residual alignment could slightly enhance ion-exchange
selectivity or precipitation is an open question.
The published literature on this subject is confusing, sometimes
contradictory, and often unreliable. There is much anecdotal
evidence about changes in the crystallization and precipitation
of calcium carbonate in the presence of a magnetic field, but
it is more difficult to find well-controlled and well-documented
repeatable studies.
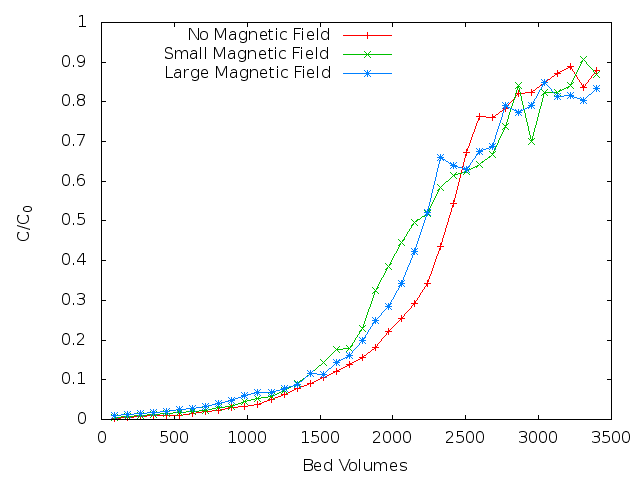
If the magnetic field has any effect at all, it would likely be quite
small. However, the ion exchange system used by Prof. Kney is quite
sensitive and offers an opportunity to make a high-quality contribution
to this field, whatever the outcome.
With support from the National Science Foundation, we were able to
show that there is little or no effect,
at least to within the sensitivity of our measurements.
The results were published in
A. K. Kney, J. Tavakoli, A. Dougherty,
``Unique Approach to
Understanding the Mechanisms involved in Magnetic
Water Conditioning using Ion Exchangers,''
Proceedings for the
1st IWA Conference on Scaling and Corrosion in Water
and Wastewater Systems, Cranfield, Bedfordshire, UK, (March
2003)
and
A. K. Kney, J. Molek, J. Tavakoli, A. Dougherty,
``The Effect of Magnetic Susceptibility on Ion Exchange
Treatment of a Solution in the Presence of a Magnetic Field,''
Proceeding for Industrial Water Conference,
Orlando, FL, CD Format (2002).
To more directly evaluate claims related to precipitation of calcium
carbonate in a magnetic field, we looked directly at the process of
precipitation in a magnetic field. Prof. Keny was able to show that
extraordinarily careful experimental protocols are required to ensure
reproducibility. Among the important factors to consider are pH,
cleaning techniques (nucleation processes are significantly affected by
trace particles left on the sample cell walls), and temperature.
Once those variables are accounted for, we found no measurable
affect on precipitation due to the magnetic field.

This page is occasionally maintained by
Andrew Dougherty