Problem 6.1, 6.5, and 6.12.
Problem 6.10, 6.18, 6.20 (skip (d) and (e)), and 6.21.
Hints:
-
For Problem 6.1, instead of plotting the total multiplicity, just plot the probability for each state as a function of qA. (The probability is simply the multiplicity divided by the total number of microstates for 500 energy units distributed among all 101 particles.) We will see that the probability follows an exponential decay. On a semilog plot, the probability will thus decay as a straight line. It is not required here, but if you wanted to compute the scaling factor, you could refer back to the take-home portion of the first test. There, you considered an Einstein solid with energy U = qϵ, and showed that
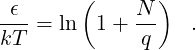
-
For Problem 6.5, the math is simpler if you use the approximation that kT ≈
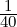 eV and note that the given energies are all multiples of
eV and note that the given energies are all multiples of 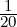 eV.
eV.
-
For Problem 6.18, note that the heat capacity is simply
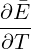 .
.
-
For Problem 6.20, note that parts (d) and (e) were included on the first take-home test.
-
For Problem 6.21, Mathematica’s ability to do symbolic calculations is very helpful. For example, suppose you had a trivial system with two energy levels, E1 and E2. You could have Mathematica calculate the partition function and average energy easily with the following commands:
Z[\[Beta]_] := Exp[-\[Beta] E1] + Exp[-\[Beta] E2] Ebar[\[Beta]_] = -D[Z[\[Beta]], \[Beta]]/Z[\[Beta]]To express the result in terms of T rather than β, you can use a substitution rule to create a new function of temperature:
EbarT[T_] = Ebar[\[Beta]] /. \[Beta] -> 1/(k T)Then once you have the energy as a function of temperature, you can easily compute the heat capacity by taking another derivative.
In this problem the partition function is longer and more complicated, but the basic structure of the problem is the same.